- SUKL reviewed documentation on Sputnik V
- SUKL received tens of pages…. medicine usually has tens of thousands
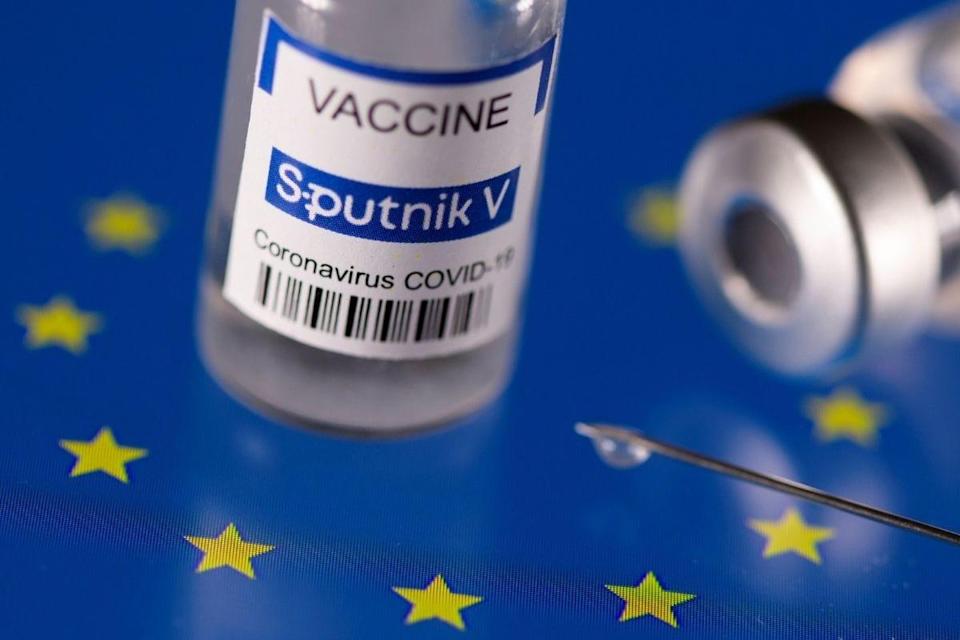
- European Medicines Agency has not authorised the Sputnik V vaccine
- President Zeman has advocated for its use
In early April, Czech Health Minister Petr Arenberger (for ANO) claimed that the unapproved vaccines, including Sputnik V, could be used in clinical trials in the Czech Republic.
“We did not receive any request for a clinical trial,,” Storova said.
She went on to say that the SUKL reviewed the documents and made comments, but that it received no input on its analysis.
According to Storova, the SUKL received a document with tens of pages, while many medicines of this kind have tens of thousands.
The European Medicines Agency (EMA) has not yet authorised the Sputnik V vaccine. President Milos Zeman of the Czech Republic has advocated for its use under a special exemption given by the Czech authorities. In his Sunday address, Zeman said that the Russian vaccine would only be used if Czechia ran out of other options.
Russia gave Czechia 300,000 Sputnik V doses, with an additional 150,000 doses delivered per month.
Jan Hamacek (Social Democrats, CSSD), the Deputy Prime Minister and Interior Minister, declared two weeks ago that he would travel to Russia to discuss the bid, but his trip was canceled.





Dear immortals, I need some wow gold inspiration to create.
Discover a world of epic quests and intense battles! Hawkplay